The first success from the collaboration with prof. Tunega
Theoretical study of adsorption on iron sulfides towards nanoparticles modeling
(RSC)
Miroslav Kolos, Daniel Tunega and František Karlický
Abstract
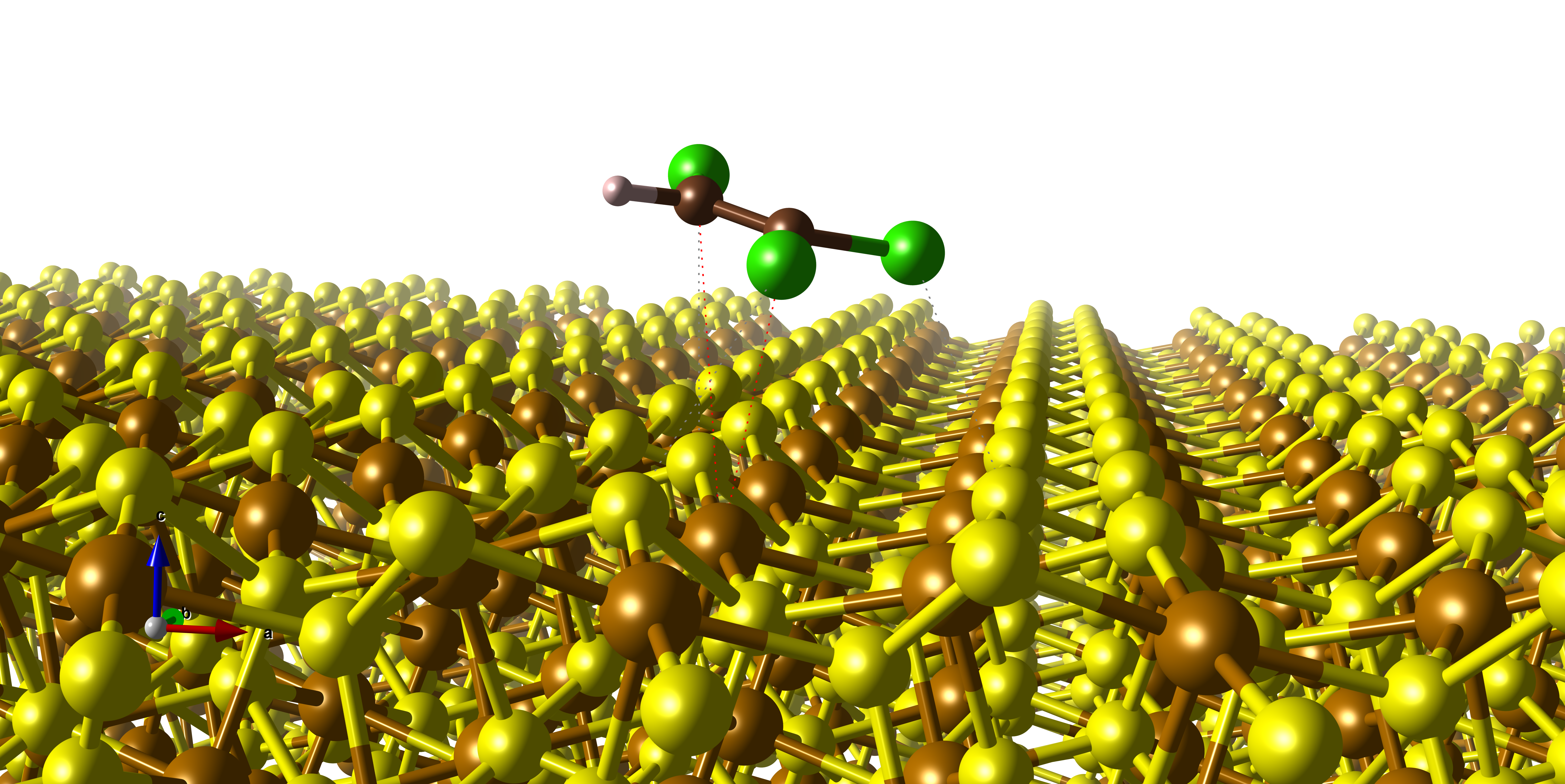
Surface modification of zero-valent iron (nZVI) nanoparticles, which are frequently used in the removal of chlorinated hydrocarbons from contaminated groundwater, can increase their surface stability without significant loss of reactivity. Sulfidation is a process, during which a thin iron sulfide phases are formed on the nZVI particles. In this work, the adsorption capability of two iron sulfide minerals (mackinawite and pyrite) and ZVI with respect to two small polar molecules (H2O and H2S) and trichloroethylene (TCE) was modeled by using the quantum mechanics (QM) approach. High-level QM methods used on cluster models served for benchmarking and validation of density functional theory methods used on periodic slab models of the (001) surfaces of iron sulfides and the (111) surface of ZVI. This careful computational treatment was necessary for achieving reliable results because modeled iron containing compounds represent computationally demanding systems. The results showed that adsorption was strongly affected by surface topology, accessibility of surface sites, and the shape of adsorbed molecular species. The mackinawite surface is practically hydrophobic having weak interactions with polar molecules (about -5/-6 kcal/mol), in contrast to the surfaces of pyrite and ZVI (adsorption energies are about three times larger). On the other hand, adsorption of weakly polar planar TCE molecule is relatively strong and similar for all three surfaces (in a range of -11 to -17 kcal/mol). Moreover, it was shown that the dominant component of the adsorption energy of TCE had origin in dispersion interactions, which were less important for small polar molecules.