Barbora Vénosová has a new paper from her collaboration with Slovak University of Technology
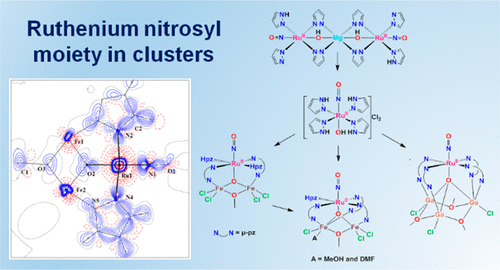
The Ruthenium Nitrosyl Moiety in Clusters: Trinuclear Linear μ-Hydroxido Magnesium(II)-Diruthenium(II), μ3-Oxido Trinuclear Diiron(III)–Ruthenium(II), and Tetranuclear μ4-Oxido Trigallium(III)-Ruthenium(II) Complexes
(ACS)
Iryna Stepanenko, Pavlo Mizetskyi, Ewelina Orlowska, Lukáš Bučinský, Michal Zalibera, Barbora Vénosová, Martin Clémancey, Geneviève Blondin, Peter Rapta, Ghenadie Novitchi, Wolfgang Schrader, Dominik Schaniel, Yu-Sheng Chen, Martin Lutz, Jozef Kožíšek, Joshua Telser, and Vladimir B. Arion
Abstract
The ruthenium nitrosyl moiety, {RuNO}6, is important as a potential releasing agent of nitric oxide and is of inherent interest in coordination chemistry. Typically, {RuNO}6 is found in mononuclear complexes. Herein we describe the synthesis and characterization of several multimetal cluster complexes that contain this unit. Specifically, the heterotrinuclear μ3-oxido clusters [Fe2RuCl4(μ3-O)(μ-OMe)(μ-pz)2(NO)(Hpz)2] (6) and [Fe2RuCl3(μ3-O)(μ-OMe)(μ-pz)3(MeOH)(NO)(Hpz)][Fe2RuCl3(μ3-O)(μ-OMe)(μ-pz)3(DMF)(NO)(Hpz)] (7·MeOH·2H2O) and the heterotetranuclear μ4-oxido complex [Ga3RuCl3(μ4-O)(μ-OMe)3(μ-pz)4(NO)] (8) were prepared from trans-[Ru(OH)(NO)(Hpz)4]Cl2 (5), which itself was prepared via acidic hydrolysis of the linear heterotrinuclear complex {[Ru(μ-OH)(μ-pz)2(pz)(NO)(Hpz)]2Mg} (4). Complex 4 was synthesized from the mononuclear Ru complexes (H2pz)[trans-RuCl4(Hpz)2] (1), trans-[RuCl2(Hpz)4]Cl (2), and trans-[RuCl2(Hpz)4] (3). The new compounds 4–8 were all characterized by elemental analysis, ESI mass spectrometry, IR, UV–vis, and 1H NMR spectroscopy, and single-crystal X-ray diffraction, with complexes 6 and 7 being characterized also by temperature-dependent magnetic susceptibility measurements and Mössbauer spectroscopy. Magnetometry indicated a strong antiferromagnetic interaction between paramagnetic centers in 6 and 7. The ability of 4 and 6–8 to form linkage isomers and release NO upon irradiation in the solid state was investigated by IR spectroscopy. A theoretical investigation of the electronic structure of 6 by DFT and ab initio CASSCF/NEVPT2 calculations indicated a redox-noninnocent behavior of the NO ancillary ligand in 6, which was also manifested in TD-DFT calculations of its electronic absorption spectrum. The electronic structure of 6 was also studied by an X-ray charge density analysis.