Skvělý úspěch z naší česko-rakouské spolupráce, nový článek v Environmental Science & Technology
Iron Nitride Nanoparticles for Enhanced Reductive Dechlorination of Trichloroethylene.
(ACS)
Miroslav Brumovský, Jana Oborná, Vesna Micić, Ondřej Malina, Josef Kašlík, Daniel Tunega, Miroslav Kolos, Thilo Hofmann, František Karlický, and Jan Filip
Abstract
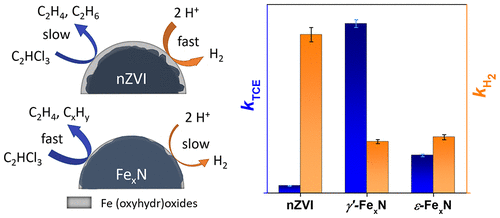
Nitriding has been used for decades to improve the corrosion resistance of iron and steel materials. Moreover, iron nitrides (FexN) have been shown to give an outstanding catalytic performance in a wide range of applications. We demonstrate that nitriding also substantially enhances the reactivity of zerovalent iron nanoparticles (nZVI) used for groundwater remediation, alongside reducing particle corrosion. Two different types of FexN nanoparticles were synthesized by passing gaseous NH3/N2 mixtures over pristine nZVI at elevated temperatures. The resulting particles were composed mostly of face-centered cubic (γ′-Fe4N) and hexagonal close-packed (ε-Fe2–3N) arrangements. Nitriding was found to increase the particles’ water contact angle and surface availability of iron in reduced forms. The two types of FexN nanoparticles showed a 20- and 5-fold increase in the trichloroethylene (TCE) dechlorination rate, compared to pristine nZVI, and about a 3-fold reduction in the hydrogen evolution rate. This was related to a low energy barrier of 27.0 kJ mol–1 for the first dechlorination step of TCE on the γ′-Fe4N(001) surface, as revealed by density functional theory calculations with an implicit solvation model. TCE dechlorination experiments with aged particles showed that the γ′-Fe4N nanoparticles retained high reactivity even after three months of aging. This combined theoretical-experimental study shows that FexN nanoparticles represent a new and potentially important tool for TCE dechlorination.